AI pathology and precision health get fresh fuel; MDR CE marks stack up; MHRA drops safety signals and an IDAP tweak—another week of pilot-to-product momentum.
People on the move

ViCentra (the Netherlands) Ex-Dexcom and Medtronic leaders join to scale Kaleido across Europe; Karen Baxter becomes SVP Sales, Europe; Jay Little becomes VP Strategy & Business Development.

Earlybird Health (Germany) Dr. Rabab Nasrallah and Dr. Christoph Massner promoted to Partners, reinforcing the firm’s biotech/medtech/data focus.
Money flows
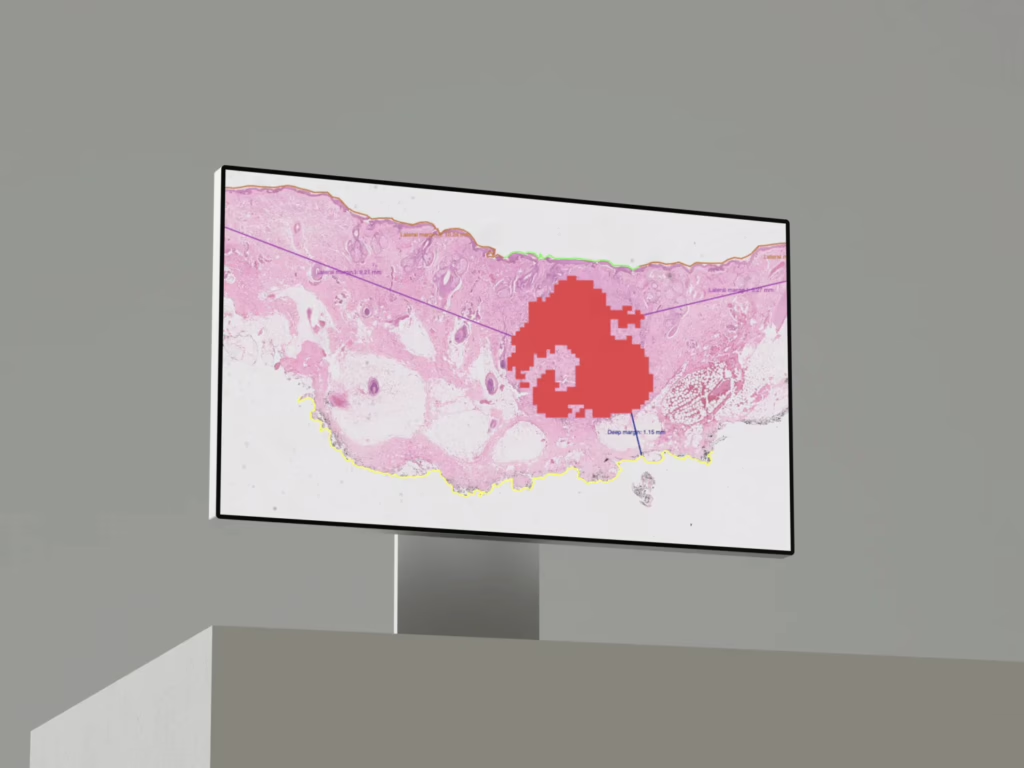
Primaa (FR)
€7M round; AI histology/cancer diagnostics. Funds support EU deployment and U.S. expansion prep.
Human Health (UK)
€4.7M Seed; patient-first precision health platform and “Human Evidence” for B2B.

MoleSense (Switzerland) — CHF 150k (€156k) Venture Kick grant; molecular maternity wearables for high-risk pregnancies.
On the press
• Lumendi EU MDR CE mark for DiLumen™ EZ¹ and DiLumen™ C¹ endotherapy devices; enables EU commercial distribution.
• Bot Image MDR CE mark for ProstatID® AI prostate MRI software, opening European market access; product is FDA-cleared in the U.S.
• MHRA — October Safety Roundup and Field Safety Notices published; useful for vigilance teams.
• MHRA — IDAP update: UCNA extension for HistoSonics’ EDISON™ ultrasound ablation system; worth tracking alongside EU MDR routes.
One thing to remember
Regulatory momentum matters: CE marks keep landing while the UK fine-tunes access pathways, and capital is returning tohiow focused clinical AI and women’s health sensors. Founders who tie clinical utility to crisp EU/UK access narratives will convert faster to distribution.
This content has been enhanced with GenAI.
