The European ecosystem is trading complexity for clarity this week: a landmark proposal to “de-clog” the MDR/IVDR bottleneck arrives alongside major CE marks in diabetes, while surgical imaging and pharmacy infrastructure secure fresh growth capital to scale.
People on the move

The French-American exoskeleton pioneer, famous for its self-balancing “Eve” system, has appointed the CEO of Permobil to its board. This move signals a hard pivot from R&D to global commercialization in the assistive mobility sector.

FiveT Fintech / Mathias Brenner & Tobias Haeckermann
The Swiss growth-stage investor has added two Growth Partners to strengthen its health-tech and fintech crossover plays, focusing on digital infrastructure in the DACH region.
Money flows
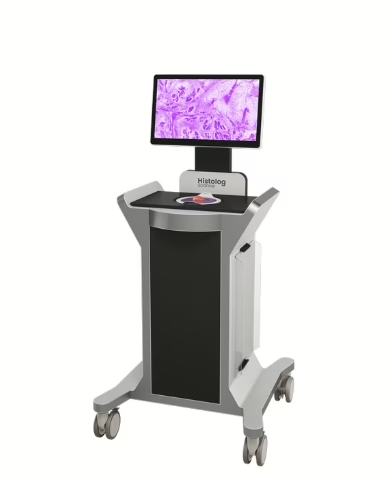
SamanTree Medical
€20M (Venture Debt/EIB),
Surgical Imaging; The European Investment Bank (EIB) is backing the Lausanne-based startup to scale its Histolog® Scanner. The device offers real-time, ultra-high-resolution imaging of tissue margins during surgery, aimed at reducing cancer re-operation rates.


Essity
€400M (EIB Loan),
Hygiene & Wound Care; The Swedish giant secured a massive RDI injection to advance bio-based materials and digital wound care solutions across its hubs in Sweden, Germany, and France through 2028.

MedVasc
€2.2M (SEK 24M), Shareholder Round,
Vascular; The Swedish startup is using the funds to progress its Solutio™ anesthesia catheter toward FDA approval, targeting a pain-free solution for laser treatment of varicose veins.
On the press
- EU Commission / MDR & IVDR Simplification — A transformative legislative proposal has been advanced to reduce administrative burdens. Key highlights include the potential reclassification of certain SaMD (Software as a Medical Device) and the introduction of a perpetual audit model to replace 5-year re-certifications.
- Senseonics / Eversense 365 CE Mark — Approval granted for the world’s first one-year implantable CGM. The EU rollout is set to begin in Germany, Italy, Spain, and Sweden, marking a major milestone in long-term diabetes management.
- MHRA / Safety & Fee Updates — The UK regulator published its January safety roundup and statutory fee changes for clinical investigations. Crucial for any EU startup with UK deployment on the 2026 roadmap.
One thing to remember
The regulatory “Great Rationalization” has begun: with the EU Commission moving to down-classify medical software and simplify audits, the cost of market entry for SaMD is poised to drop. Founders who can align their clinical evidence with these emerging, leaner pathways will have a massive advantage in the 2026 fundraising environment.
