Robotics bags big money, IVF gets an automation CE mark, and UK regulators sketch next steps for AI in care.
People on the move

Distalmotion (Switzerland) Chas McKhann becomes Executive Chairman alongside a $150M raise; focus is US growth while keeping EU base in Lausanne.
Money flows
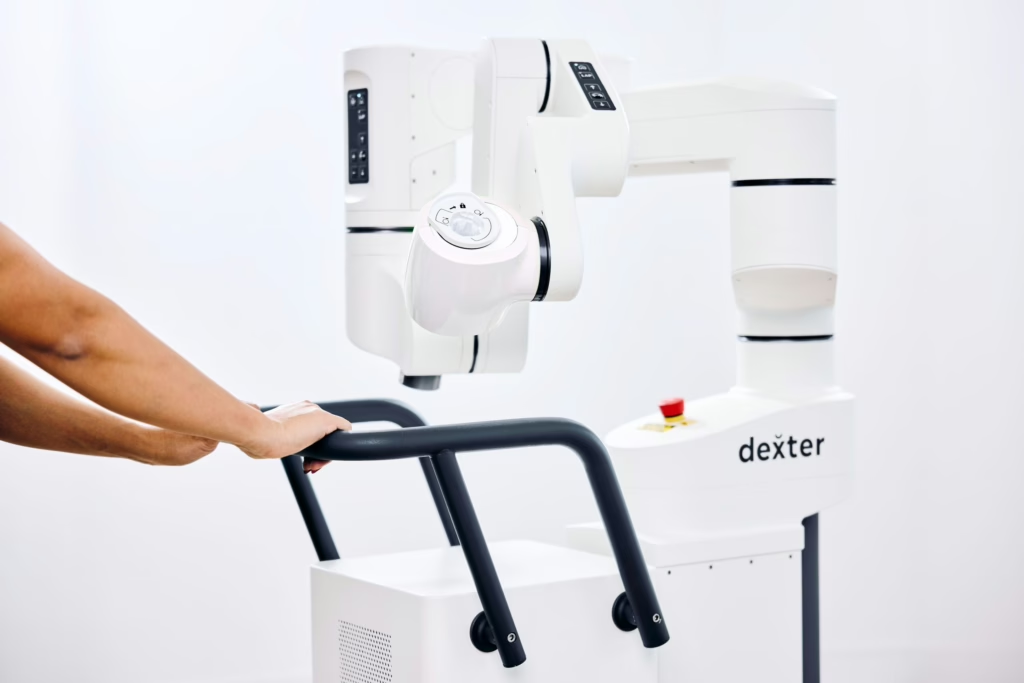
Distalmotion (Switzerland) $150M, Series G / growth; scaling DEXTER® surgical robotics with ASC push and US commercial build-out.

Sofinnova Partners (France) — €650M, flagship Capital XI; early-stage focus in medtech/biopharma, active deployment underway.
On the press
• Overture Life (Spain) CE markfor DaVitri™, billed as the first automated device cleared in the EU or US for vitrifying unfertilised eggs; EU commercial rollout begins.
• Cardiovalve (Israel) CE file submitted for transcatheter tricuspid valve after positive TARGET study interim; EU approval process initiated.
• JenaValve (Germany) 1,000th case with CE-marked Trilogy™ TAVR for aortic regurgitation/stenosis, signalling steady EU adoption.
• MHRA (UK): Professor Alastair Denniston outlines principles for “safe, fast and trusted” regulation of AI in healthcare; more detail expected in 2026.
One thing to remember
Capital and credibility still move together: big-ticket robotics funding and a heavyweight €650M early-stage fund arrived the same week that EU-relevant CE activity and the UK’s AI-in-health policy guardrails advanced. It is an evidence that clear regulatory paths plus deployment stories are what unlock cheques right now.
