A compact week: venture debt fuels vascular access, cardiology gets a non-invasive CE mark, and NICE nudges digital rehab platforms toward the NHS.
People on the move
Lucile Blaise joins LivaNova (UK) as Global Head of Commercialization, Obstructive Sleep Apnea, adding strong EU market access chops to the executive team.

Money flows
Angelini Ventures (Italy): €150M EIB co-investment agreement to back European biotech and digital health startups over six years (EIB €75M + Angelini Ventures €75M). Signals more institutional firepower for EU healthtech.

Annette (France) €2M round to scale its GLP-1 companion care platform for structured obesity support; led by Redstone, Ring Capital and AFI Ventures.
Ray Studios (France) €10M to expand its physician-led tattoo-removal clinic network across Europe; co-led by Factory Capital and Nickleby Capital.
On the press
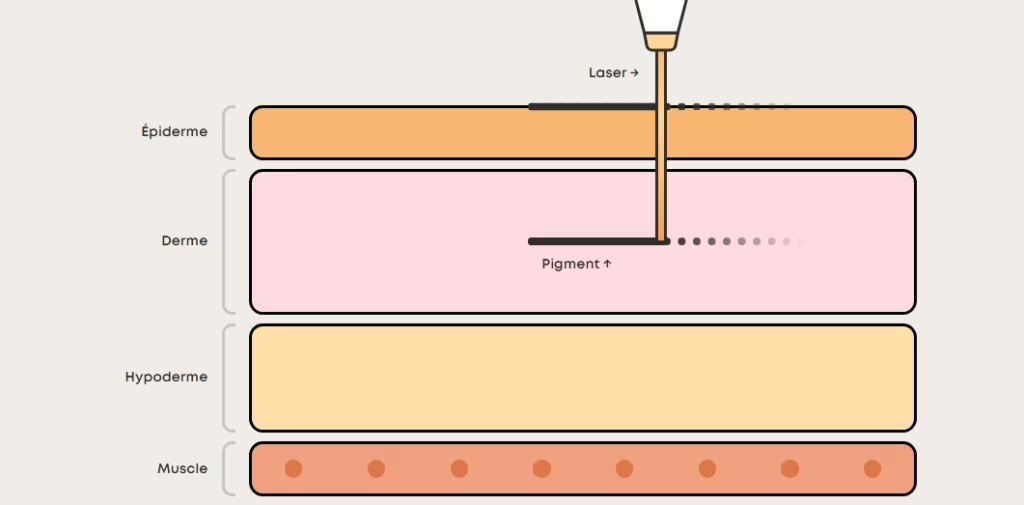
• Cardiawave (FR): Valvosoft® receives CE Mark as the first non-invasive ultrasound therapy for severe symptomatic aortic stenosis; data cited from EU FIM and pivotal studies across 12 centres.
• Boston Scientific: European approval for the Farapoint™ pulsed-field ablation focal catheter to treat atrial fibrillation, complementing Farapulse™ PFA platform.
• NICE (UK) publishes Early Value Assessment (HTE35) for digital platforms supporting cardiac rehabilitation, enabling conditional NHS use while evidence is generated over three years.
One thing to remember
EU cardiology is having a moment: capital is flowing into commercialization-ready devices, while regulators opened the door to earlier adoption of digital rehab and cleared a novel non-invasive therapy. Founders who can pair strong clinical data with payer-relevant outcomes will find both financing and fast-track pathways this winter.
