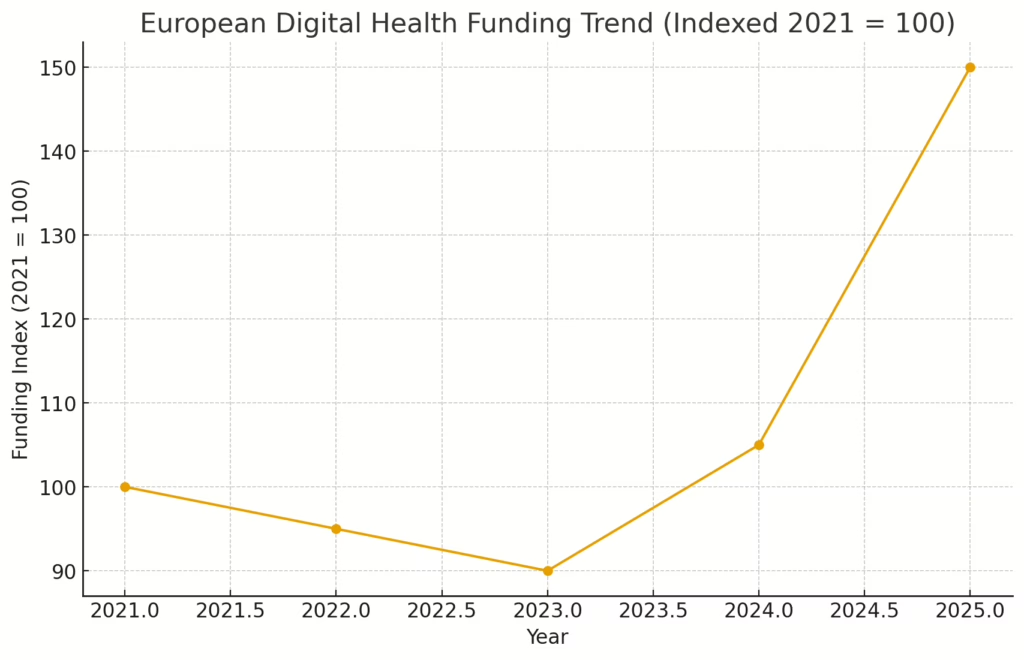
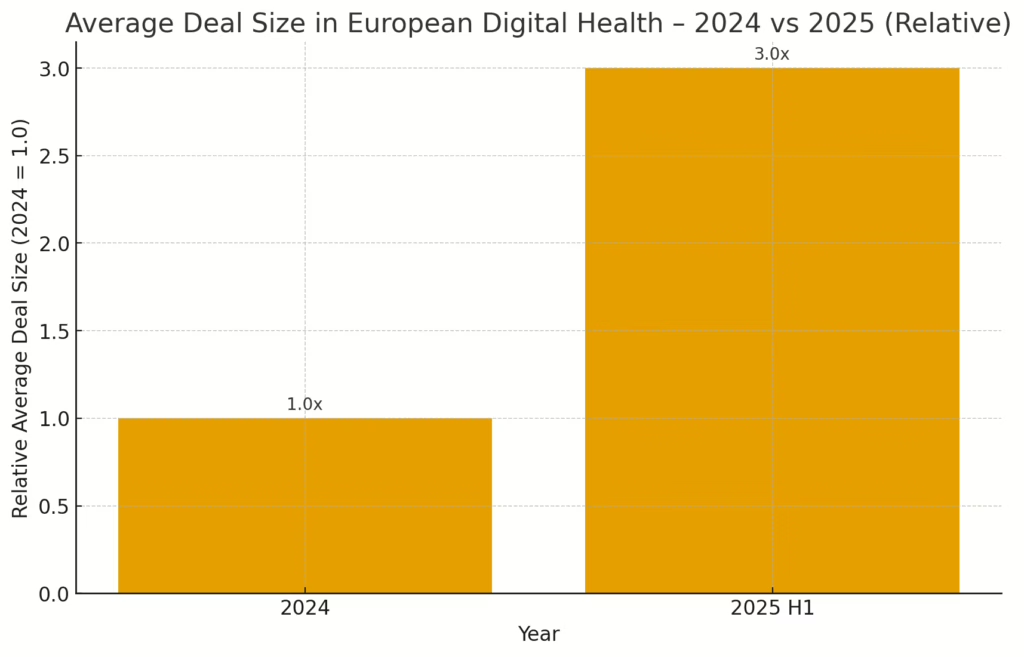
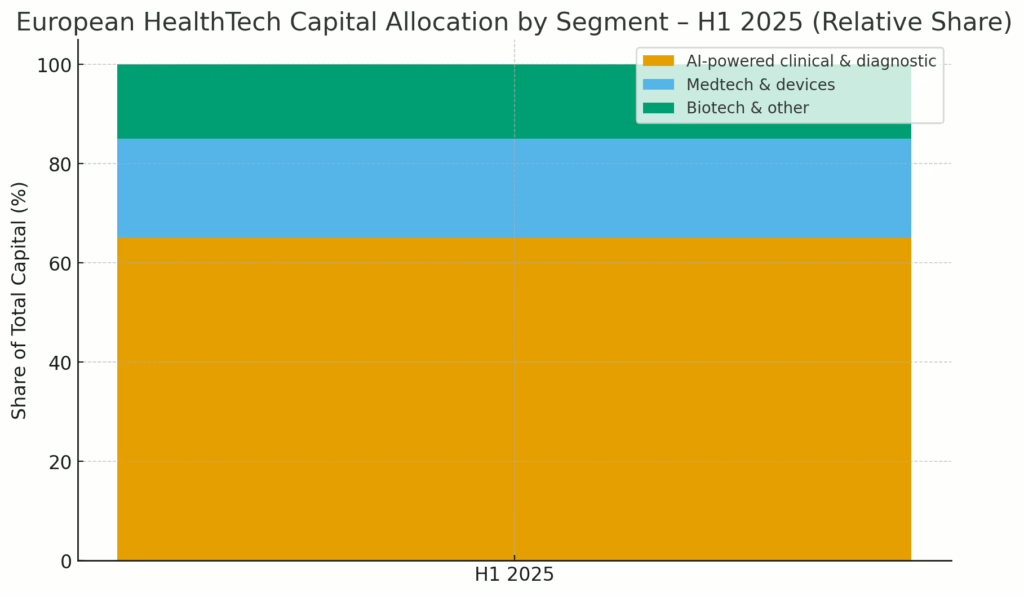
Oncology operating systems, perioperative risk AI and pre-CT stroke triage all raised fresh capital this week, while Brussels and Munich quietly tightened the screws on AI compliance and imaging vendors celebrated a shiny new CE mark.
People on the move

Ergea Group (Luxembourg)
New CEO for pan-European imaging & cancer care
Ergéa, a Luxembourg-based pan-European provider of diagnostic imaging and cancer care services, has promoted David Rolfe from UK CEO to Group CEO and appointed Mark Graves as the new CEO of Ergéa UK, signalling a more integrated European growth push in imaging and radiotherapy infrastructure.
Restore Medical (Israel):
ex-Medtronic dealmaker takes the chair
Heart-failure device company Restore Medical (Israel, backed in part by the European Innovation Council Fund) has appointed Chris Cleary, former SVP Corporate Development at Medtronic and architect of the Covidien mega-deal, as chairman of the board to guide its transcatheter heart-failure therapy through advanced US and global clinical development.

Money flows

Gosta Labs (Finland)
€7.5M seed, oncology AI operating system
Helsinki-based Gosta Labs raised a €7.5 million seed round, led by Voima Ventures with COR Group, the Aho family, Reaktor and other angels, to scale its oncology-focused AI operating system that turns each patient encounter into structured data, slashes documentation time and links care decisions to international treatment guidelines. The company plans to deepen medical-device-grade development and expand deployments across Europe, the Baltics and Australia.
Noteless (Norway)
€3.5M to fight doctor burnout
Oslo-based Noteless, a Norwegian HealthTech startup automating clinical documentation and task management, closed a €3.5 million round to tackle physician burnout by cutting admin time in hospitals and clinics; the investor syndicate includes People Ventures and Alliance Venture, with the company targeting broader Nordic and European rollout.


Healthplus.ai (NL)
€2.3M late-seed for perioperative risk prediction AI
Amsterdam-based Healthplus.ai raised €2.3 million in a late-seed round led by Elevating Capital and co-led by LUMO Labs, with ROM InWest and Pathena among the participants, to expand PERISCOPE®, its ISO- and CE-certified AI system that predicts post-surgical infection risk and suggests mitigation strategies for surgical teams. The funding will support deeper EHR integrations (Epic, Cerner, ChipSoft), further model development across perioperative pathways and broader roll-out in Europe plus FDA clearance work for the US.
AI-Stroke (France)
$4.6M seed for pre-CT stroke triage
Paris-area medtech AI-Stroke secured a US$4.6 million seed round led by Heka (Newfund VC) with Bpifrance and angels, to advance an “AI neurologist” that runs on smartphones or tablets for pre-hospital stroke triage, analysing 30-second video of facial symmetry, arm movement and speech. The capital will fund FDA regulatory work and multisite clinical studies in leading US stroke centres, with the company also adding a heavyweight international stroke advisory board.

On the press
TÜV SÜD launches voluntary EU AI Act conformity certificates
TÜV SÜD announced new services for early, voluntary conformity certificates under the EU AI Act (Regulation (EU) 2024/1689), offering manufacturers of AI systems including high-risk use cases in medicine and medical devices an independent review of technical documentation and partial compliance ahead of mandatory assessments. The program covers gap assessments, training and an “Attestation of Conformity,” aimed at helping companies get AI products AI-Act-ready before notified bodies are fully designated.
GE HealthCare wins CE mark for Omni 128 cm total-body PET/CT
GE HealthCare received CE mark for its Omni 128 cm total-body PET/CT system, enabling commercialisation in the EU of an ultra-long axial field-of-view scanner intended to improve sensitivity, enable low-dose protocols and support advanced oncology and theranostics workflows. The platform is pitched at high-throughput cancer centres and research hubs across Europe.
MHRA outlines “innovative approaches” to medtech regulatory reform
The UK MHRA’s MedRegs blog set out its latest thinking on medtech regulatory reform, highlighting more agile statutory instruments, innovation-friendly approval routes, and closer alignment with international partners for medical devices and IVDs. For EU-facing companies selling into the UK, the piece is a useful signal on future reliance routes and how sandbox-style approaches may coexist with post-market vigilance expectations.
One thing to remember
AI-heavy clinical tools are still getting funded from oncology operating systems to perioperative risk prediction and pre-CT stroke triage. This week’s TÜV SÜD and MHRA moves are a clear reminder that in Europe, “AI-first” now has to mean “AI-and-compliance-first.”
For founders, the competitive edge is shifting toward teams that can show device-grade evidence and be visibly AI-Act-ready long before their product hits a hospital PACS or EHR.
This content has been enhanced with GenAI.